How to guide: Assessing, arranging and confirming capability and capacity
This guide outlines the ways in which practices should undertake the appropriate Assess, Arrange and Confirm Capacity and Capability stage of site agreement when setting up a study.
Capacity and capability (C&C) assessment is the method by which sites consider whether they can take on a new study. In some cases this needs to be a formal assessment and confirmation, in other cases an email may suffice, or in others the Health Research Authority (HRA) may state that the study can proceed without C&C unless the site indicates any objection. Regarding C&C for Participant Identification Centre (PIC) activity, please see the ‘Understanding the role of PICs’ guide, and the below PIC section.
See also HRA guidance on: Assess, arrange and confirm terminology.
Key information you should be aware of:
- South West London Research & Development issues an advisory email for studies of which we have had sight, to assist practices with completing their review. Practices can request a copy of this advisory from SWL R&D or from the study team.
- Research governance is conducted centrally by the HRA. The HRA issues HRA approval which incorporates ethical review.
- Each participating site must assess, arrange and confirm their capability and capacity to participate, dependent on the requirements of the individual study.
- The decision to participate, and the agreement, should be at the level of the participating organisation, i.e. usually at the level of the practice (as an independent contractor). PCNs are not able to sign a contract unless they have been set up as a company, and neither a PCN nor a federation is not able to sign a contract on behalf of its members unless there is a formal agreement that they can do this. If the research is taking place at the federation level, e.g. in the out of hours service, then this could be signed off by the federation. The ICB cannot sign off on behalf of practices.
- It is important to note that the processes confirmed in the HRA approval letter must be followed, as formal confirmation or signing of agreements is legally necessary where indicated in this letter.
Full guide
Pre-HRA approval
- Sponsors are encouraged to liaise with potential sites as early as possible in the process of developing their study. This may be before HRA approval for the study has been received or even applied for.
- At the pre-approval stage, sites can carry out a feasibility review of the study documents, but should be aware that the documents cannot be considered final until HRA approval is confirmed to the study team.
- A patient could be involved to discuss study feasibility, but no patient recruitment can usually take place until the study is approved.
After HRA approval of the study
- The next step in the process is that the study team will receive a HRA approval letter.
- When the HRA approval letter is received, it will confirm expectations related to confirmation of capacity and capability (in the ‘Information to support study setup’ table at the end of the letter).
- This will include stating whether formal confirmation of C&C (capability and capacity) is required, and whether the study intends to use the Organisation Information Document (OID) as the site agreement.
- You should ask for a copy of the letter and check this table first to inform what you need to do next.
- South West London R&D should also have the opportunity to issue an advisory email which will give advice on next steps in assessing, arranging and confirming C&C.
- If formal confirmation of C&C is required, see below for detail of the steps.
How to assess
Further to any initial feasibility assessment done at the Expression of Interest (EOI) stage, this is the main and formal feasibility assessment. When formally confirming your capacity & capability, and/or confirming the OID or contract, this is where you commit to the study, so this assessment should be more in-depth. Depending on the study commitment, this may be quick (for example if just a staff survey), or may require more consideration.
At this stage, you should have from the study team:
- A copy of the study protocol and local documents (the Local Information Pack).
- A copy of the Organisation Information Document or study contract.
An advisory email will have been sent to the study team from SWL R&D, and you can request a copy of this from the study team or from SWL R&D.
At the ‘assess’ stage you should review the study protocol and/or OID/contract and consider the following:
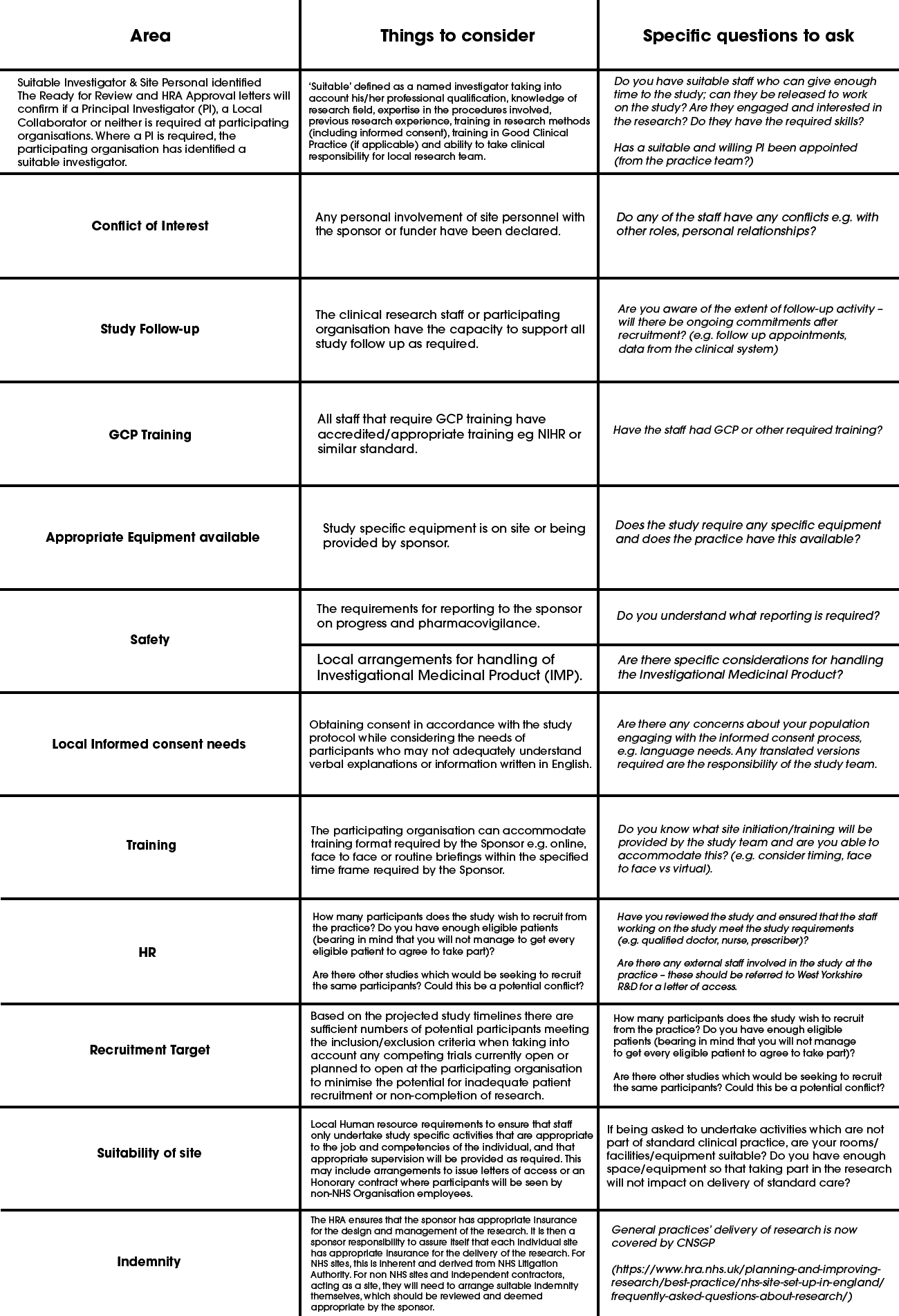
Guidance [first two columns] provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf<
How to arrange
At this stage, the practice should put any necessary arrangements in place to deliver the study. Even when studies don’t require formal confirmation of C&C, there may be some arrangement required.
Please note that, if the study requires software to be added to the practice computers, this usually requires involvement from IT and you can seek advice from South West London R&D.
The practice should consider each of the following arrangements, although these will vary by study:
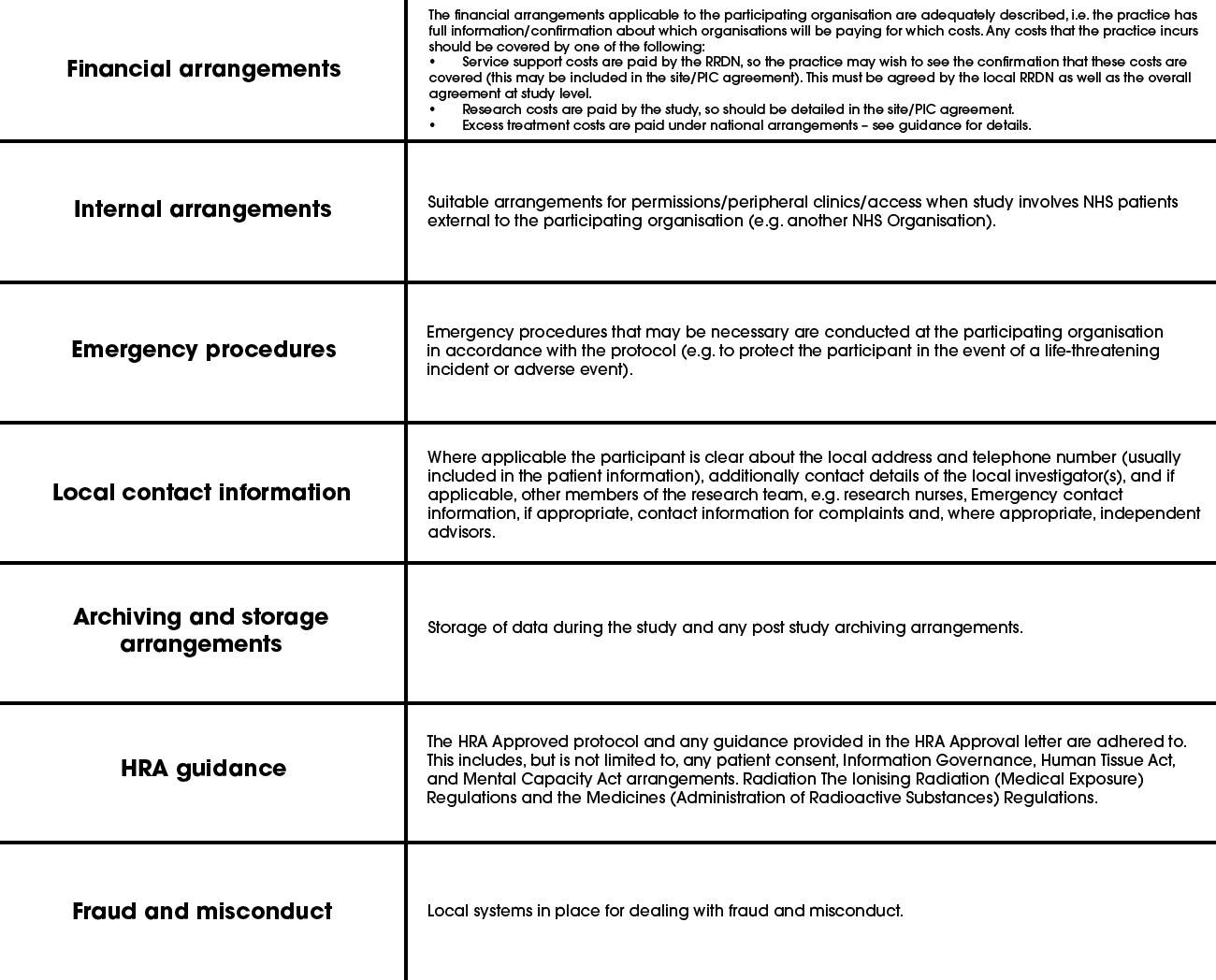
Guidance provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf)
How to confirm
At this stage, the practice confirms its participation (and confirms they are ready to start). The ways of doing this are varied depending on the study – this is confirmed in the HRA approval letter.
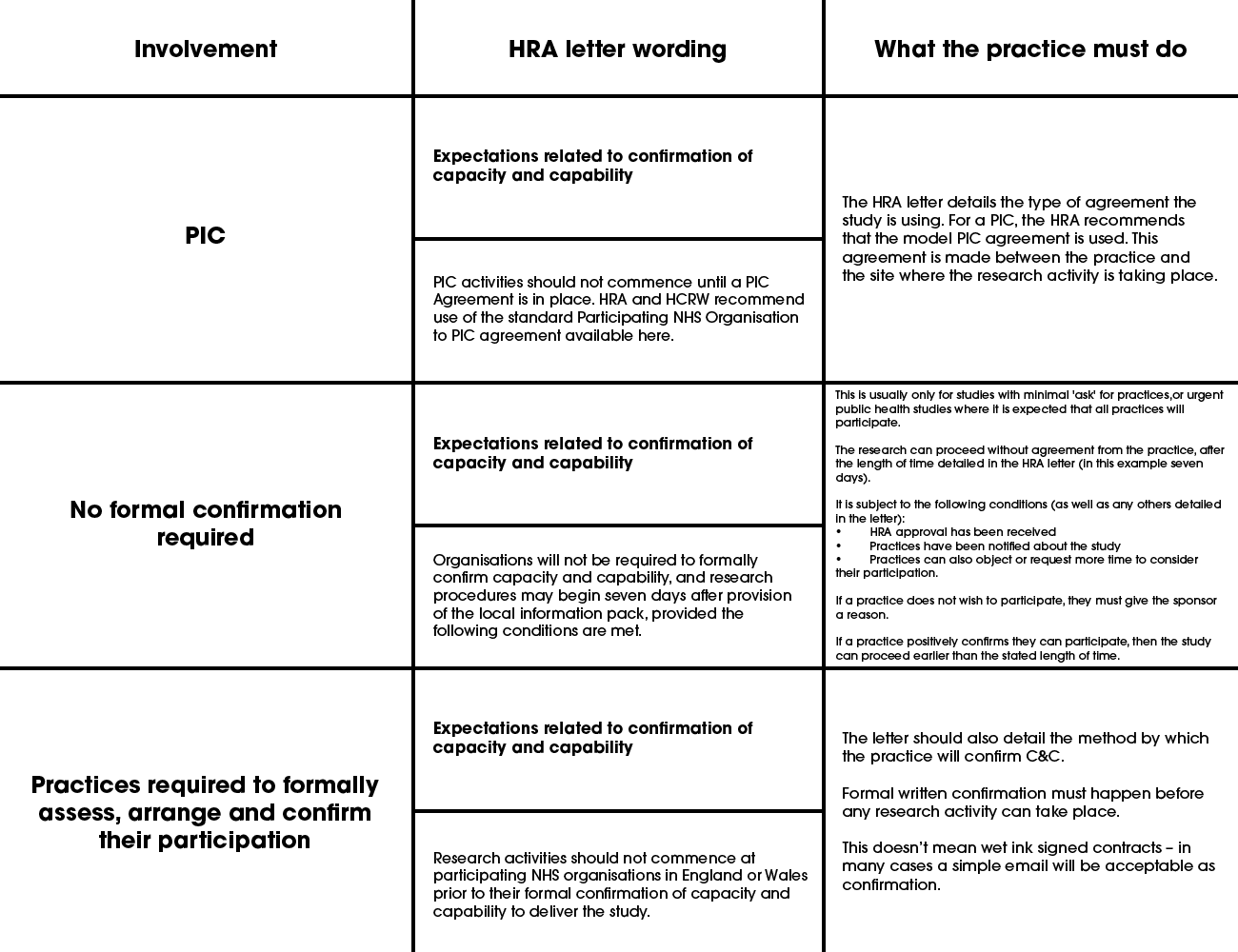
The next column in the table indicates the agreement to be used to confirm participation. See also the guide for contracts. Although the OID or the model agreement are the preferred contracts, you will need to use the contract which has been submitted and agreed by the HRA.
Most commonly, the standard agreements are used:
- An Organisation Information Document has been submitted and the sponsor is not requesting and does not expect any other site agreement to be used.
An authorised member of staff from the practice should check through the Localised Organisation Information Document (OID). This should detail what is required by the study, and all of the fields marked with a * should be completed by the study team, outlining the requirements. This should include start and finish dates, recruitment target, equipment and training requirements. The appendices detail any financial provisions, how materials will be shared and data processing/sharing arrangements.
Items marked with a ^ are to be completed by the practice; then the OID should be returned by email from an authorised signatory (wet ink signatures are not expected).
The Local OID is not a final document presented to the practice, but is to be negotiated and agreed between the practice and the study. Here is where you can agree targets, finances and local arrangements.
An unmodified site agreement can also be used:
- Organisational Information Documents and respective Schedules of Events have been submitted for each site type; the sponsor is also intending to use a separate site agreements. The agreement is unmodified.
- HRA and HCRW recommend use of the standard Participating NHS Organisation to PIC agreement.
Unmodified agreements have been negotiated and reviewed by legal teams centrally, so practices can be assured that they can sign up to these, provided they have reviewed and agree to the study detail in the appendices.
The PIC agreement is appropriate when acting as a Participant Identification Centre (PIC) [see Understanding the role of PICs guide]. It is an agreement between the practice and the research site (often a hospital trust).
The person signing the agreement must be an appropriately delegated member of staff from the practice.
An electronic signature will often be acceptable with agreement from the Sponsor.
The signed copy should be kept in the site file (either hard copy or electronic).
Modified/bespoke agreements are not recommended but can be used if agreed by the HRA:
- An organisation information document has been submitted, and the sponsor is intending to use a separate site agreement. The sponsor is using a bespoke agreement. The HRA and HCRW take no position on the acceptability of these changes. Participating NHS organisations should now determine its acceptability and liaise with the sponsor to confirm the content of the agreement.
It should be specified in the HRA letter where the Sponsor has used a modified or bespoke agreement.
Where non-standard/modified agreements are used, the practice may wish to seek legal advice, but at the very least should carefully check through the agreement terms to make sure they are happy with these.
Sometimes, different parts of the study are subject to different agreements.
- An Organisation Information Document has been submitted for Phases A and B and the sponsor is not requesting and does not expect any other site agreement to be used.
- PICs – Sponsor has confirmed that a relevant PIC agreement will be in place between the PIC and the NHS research site they will act as PIC’s for. Research sites – An Organisation Information Document has been submitted and the sponsor is intending to use a separate site agreement.

When different parts of the study are mentioned, the practice should confirm which parts of the study are applicable to them.
The detail should be found in the OID, but if not sure you should clarify with the study team and receive confirmation in writing where possible.
Other document types
Studies which started a number of years ago may try to use older documents. The SSI (Site specific information) and Statement of Activities are now out of date so can no longer be used for confirming participation. The study team should be directed to https://www.myresearchproject.org.uk/help/hlpsitespecific.aspx#UK-Local-Information-Pack-Transition to replace these documents with the current ones.
Further advice and examples of setting up studies in primary care:
You can read more about study setup in primary care settings, including examples, at https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/HRA_Approval_in_Primary_Care_Settings__Principles_of_Study_Set-Up_Version_2.0_09Mar17.pdf (note that this document contains some old references but can be taken as the most recent guidance).
Glossary of Acronyms and Terms
-
- C&C – Capacity and Capability
- RDN – Research Delivery Network
- HRA – Health Research Authority
- OID – Organisation Information Document
- PI – Principal Investigator
- PIC – Participant Identification Centre
- SSI – Site Specific Information (form)
Pre-HRA approval
- Sponsors are encouraged to liaise with potential sites as early as possible in the process of developing their study. This may be before HRA approval for the study has been received or even applied for.
- At the pre-approval stage, sites can carry out a feasibility review of the study documents, but should be aware that the documents cannot be considered final until HRA approval is confirmed to the study team.
- A patient could be involved to discuss study feasibility, but no patient recruitment can usually take place until the study is approved.
- Sponsors are encouraged to liaise with potential sites as early as possible in the process of developing their study. This may be before HRA approval for the study has been received or even applied for.
- At the pre-approval stage, sites can carry out a feasibility review of the study documents, but should be aware that the documents cannot be considered final until HRA approval is confirmed to the study team.
- A patient could be involved to discuss study feasibility, but no patient recruitment can usually take place until the study is approved.
After HRA approval of the study
- The next step in the process is that the study team will receive a HRA approval letter.
- When the HRA approval letter is received, it will confirm expectations related to confirmation of capacity and capability (in the ‘Information to support study setup’ table at the end of the letter).
- This will include stating whether formal confirmation of C&C (capability and capacity) is required, and whether the study intends to use the Organisation Information Document (OID) as the site agreement.
- You should ask for a copy of the letter and check this table first to inform what you need to do next.
- South West London R&D should also have the opportunity to issue an advisory email which will give advice on next steps in assessing, arranging and confirming C&C.
- If formal confirmation of C&C is required, see below for detail of the steps.
- The next step in the process is that the study team will receive a HRA approval letter.
- When the HRA approval letter is received, it will confirm expectations related to confirmation of capacity and capability (in the ‘Information to support study setup’ table at the end of the letter).
- This will include stating whether formal confirmation of C&C (capability and capacity) is required, and whether the study intends to use the Organisation Information Document (OID) as the site agreement.
- You should ask for a copy of the letter and check this table first to inform what you need to do next.
- South West London R&D should also have the opportunity to issue an advisory email which will give advice on next steps in assessing, arranging and confirming C&C.
- If formal confirmation of C&C is required, see below for detail of the steps.
How to assess
Further to any initial feasibility assessment done at the Expression of Interest (EOI) stage, this is the main and formal feasibility assessment. When formally confirming your capacity & capability, and/or confirming the OID or contract, this is where you commit to the study, so this assessment should be more in-depth. Depending on the study commitment, this may be quick (for example if just a staff survey), or may require more consideration.
At this stage, you should have from the study team:
- A copy of the study protocol and local documents (the Local Information Pack).
- A copy of the Organisation Information Document or study contract.
An advisory email will have been sent to the study team from SWL R&D, and you can request a copy of this from the study team or from SWL R&D.
At the ‘assess’ stage you should review the study protocol and/or OID/contract and consider the following:

Guidance [first two columns] provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf<
Further to any initial feasibility assessment done at the Expression of Interest (EOI) stage, this is the main and formal feasibility assessment. When formally confirming your capacity & capability, and/or confirming the OID or contract, this is where you commit to the study, so this assessment should be more in-depth. Depending on the study commitment, this may be quick (for example if just a staff survey), or may require more consideration.
At this stage, you should have from the study team:
- A copy of the study protocol and local documents (the Local Information Pack).
- A copy of the Organisation Information Document or study contract.
An advisory email will have been sent to the study team from SWL R&D, and you can request a copy of this from the study team or from SWL R&D.
At the ‘assess’ stage you should review the study protocol and/or OID/contract and consider the following:

Guidance [first two columns] provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf<
How to arrange
At this stage, the practice should put any necessary arrangements in place to deliver the study. Even when studies don’t require formal confirmation of C&C, there may be some arrangement required.
Please note that, if the study requires software to be added to the practice computers, this usually requires involvement from IT and you can seek advice from South West London R&D.
The practice should consider each of the following arrangements, although these will vary by study:

Guidance provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf)
At this stage, the practice should put any necessary arrangements in place to deliver the study. Even when studies don’t require formal confirmation of C&C, there may be some arrangement required.
Please note that, if the study requires software to be added to the practice computers, this usually requires involvement from IT and you can seek advice from South West London R&D.
The practice should consider each of the following arrangements, although these will vary by study:

Guidance provided by the NIHR Clinical Research Network (now Research Delivery Network: RDN) (https://www.rdforum.nhs.uk/content/wp-content/uploads/2015/10/UTF-8CRN-principles-for-local-capacity-and-capa.pdf)
How to confirm
At this stage, the practice confirms its participation (and confirms they are ready to start). The ways of doing this are varied depending on the study – this is confirmed in the HRA approval letter.

The next column in the table indicates the agreement to be used to confirm participation. See also the guide for contracts. Although the OID or the model agreement are the preferred contracts, you will need to use the contract which has been submitted and agreed by the HRA.
Most commonly, the standard agreements are used:
- An Organisation Information Document has been submitted and the sponsor is not requesting and does not expect any other site agreement to be used.
An authorised member of staff from the practice should check through the Localised Organisation Information Document (OID). This should detail what is required by the study, and all of the fields marked with a * should be completed by the study team, outlining the requirements. This should include start and finish dates, recruitment target, equipment and training requirements. The appendices detail any financial provisions, how materials will be shared and data processing/sharing arrangements.
Items marked with a ^ are to be completed by the practice; then the OID should be returned by email from an authorised signatory (wet ink signatures are not expected).
The Local OID is not a final document presented to the practice, but is to be negotiated and agreed between the practice and the study. Here is where you can agree targets, finances and local arrangements.
An unmodified site agreement can also be used:
- Organisational Information Documents and respective Schedules of Events have been submitted for each site type; the sponsor is also intending to use a separate site agreements. The agreement is unmodified.
- HRA and HCRW recommend use of the standard Participating NHS Organisation to PIC agreement.
Unmodified agreements have been negotiated and reviewed by legal teams centrally, so practices can be assured that they can sign up to these, provided they have reviewed and agree to the study detail in the appendices.
The PIC agreement is appropriate when acting as a Participant Identification Centre (PIC) [see Understanding the role of PICs guide]. It is an agreement between the practice and the research site (often a hospital trust).
The person signing the agreement must be an appropriately delegated member of staff from the practice.
An electronic signature will often be acceptable with agreement from the Sponsor.
The signed copy should be kept in the site file (either hard copy or electronic).
Modified/bespoke agreements are not recommended but can be used if agreed by the HRA:
- An organisation information document has been submitted, and the sponsor is intending to use a separate site agreement. The sponsor is using a bespoke agreement. The HRA and HCRW take no position on the acceptability of these changes. Participating NHS organisations should now determine its acceptability and liaise with the sponsor to confirm the content of the agreement.
It should be specified in the HRA letter where the Sponsor has used a modified or bespoke agreement.
Where non-standard/modified agreements are used, the practice may wish to seek legal advice, but at the very least should carefully check through the agreement terms to make sure they are happy with these.
Sometimes, different parts of the study are subject to different agreements.
- An Organisation Information Document has been submitted for Phases A and B and the sponsor is not requesting and does not expect any other site agreement to be used.
- PICs – Sponsor has confirmed that a relevant PIC agreement will be in place between the PIC and the NHS research site they will act as PIC’s for. Research sites – An Organisation Information Document has been submitted and the sponsor is intending to use a separate site agreement.

When different parts of the study are mentioned, the practice should confirm which parts of the study are applicable to them.
The detail should be found in the OID, but if not sure you should clarify with the study team and receive confirmation in writing where possible.
At this stage, the practice confirms its participation (and confirms they are ready to start). The ways of doing this are varied depending on the study – this is confirmed in the HRA approval letter.

The next column in the table indicates the agreement to be used to confirm participation. See also the guide for contracts. Although the OID or the model agreement are the preferred contracts, you will need to use the contract which has been submitted and agreed by the HRA.
Most commonly, the standard agreements are used:
- An Organisation Information Document has been submitted and the sponsor is not requesting and does not expect any other site agreement to be used.
An authorised member of staff from the practice should check through the Localised Organisation Information Document (OID). This should detail what is required by the study, and all of the fields marked with a * should be completed by the study team, outlining the requirements. This should include start and finish dates, recruitment target, equipment and training requirements. The appendices detail any financial provisions, how materials will be shared and data processing/sharing arrangements.
Items marked with a ^ are to be completed by the practice; then the OID should be returned by email from an authorised signatory (wet ink signatures are not expected).
The Local OID is not a final document presented to the practice, but is to be negotiated and agreed between the practice and the study. Here is where you can agree targets, finances and local arrangements.
An unmodified site agreement can also be used:
- Organisational Information Documents and respective Schedules of Events have been submitted for each site type; the sponsor is also intending to use a separate site agreements. The agreement is unmodified.
- HRA and HCRW recommend use of the standard Participating NHS Organisation to PIC agreement.
Unmodified agreements have been negotiated and reviewed by legal teams centrally, so practices can be assured that they can sign up to these, provided they have reviewed and agree to the study detail in the appendices.
The PIC agreement is appropriate when acting as a Participant Identification Centre (PIC) [see Understanding the role of PICs guide]. It is an agreement between the practice and the research site (often a hospital trust).
The person signing the agreement must be an appropriately delegated member of staff from the practice.
An electronic signature will often be acceptable with agreement from the Sponsor.
The signed copy should be kept in the site file (either hard copy or electronic).
Modified/bespoke agreements are not recommended but can be used if agreed by the HRA:
- An organisation information document has been submitted, and the sponsor is intending to use a separate site agreement. The sponsor is using a bespoke agreement. The HRA and HCRW take no position on the acceptability of these changes. Participating NHS organisations should now determine its acceptability and liaise with the sponsor to confirm the content of the agreement.
It should be specified in the HRA letter where the Sponsor has used a modified or bespoke agreement.
Where non-standard/modified agreements are used, the practice may wish to seek legal advice, but at the very least should carefully check through the agreement terms to make sure they are happy with these.
Sometimes, different parts of the study are subject to different agreements.
- An Organisation Information Document has been submitted for Phases A and B and the sponsor is not requesting and does not expect any other site agreement to be used.
- PICs – Sponsor has confirmed that a relevant PIC agreement will be in place between the PIC and the NHS research site they will act as PIC’s for. Research sites – An Organisation Information Document has been submitted and the sponsor is intending to use a separate site agreement.

When different parts of the study are mentioned, the practice should confirm which parts of the study are applicable to them.
The detail should be found in the OID, but if not sure you should clarify with the study team and receive confirmation in writing where possible.
Other document types
Studies which started a number of years ago may try to use older documents. The SSI (Site specific information) and Statement of Activities are now out of date so can no longer be used for confirming participation. The study team should be directed to https://www.myresearchproject.org.uk/help/hlpsitespecific.aspx#UK-Local-Information-Pack-Transition to replace these documents with the current ones.
Further advice and examples of setting up studies in primary care:
You can read more about study setup in primary care settings, including examples, at https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/HRA_Approval_in_Primary_Care_Settings__Principles_of_Study_Set-Up_Version_2.0_09Mar17.pdf (note that this document contains some old references but can be taken as the most recent guidance).
Glossary of Acronyms and Terms
-
- C&C – Capacity and Capability
- RDN – Research Delivery Network
- HRA – Health Research Authority
- OID – Organisation Information Document
- PI – Principal Investigator
- PIC – Participant Identification Centre
- SSI – Site Specific Information (form)