How to guide: Finance
This guide covers everything finance, from who pays to obtaining the finance, as well as other options available.
Highlights
Key information you should be aware of:
It can be difficult to understand how studies are funded, what practices will be paid, and how. Funding can also come from multiple sources.
- Practices should make themselves aware of what finance is available and what they need to do to obtain this.
- Practices may need to put contracts in place to enable funding to be paid.
- Study teams should clearly outline payments.
- Some payments may be variable depending on how many participants are recruited or on the number of mailout letters/texts sent.
- Other funding may also be available to support practices.
- NHS treatment costs or excess treatment costs are paid up front by practices but then paid back in full.
Full guide
Who pays
Research guidance is clear on which organisation is responsible for paying for certain things.
You will be given information on these, but the below is provided for information to aid understanding about who is responsible for what.
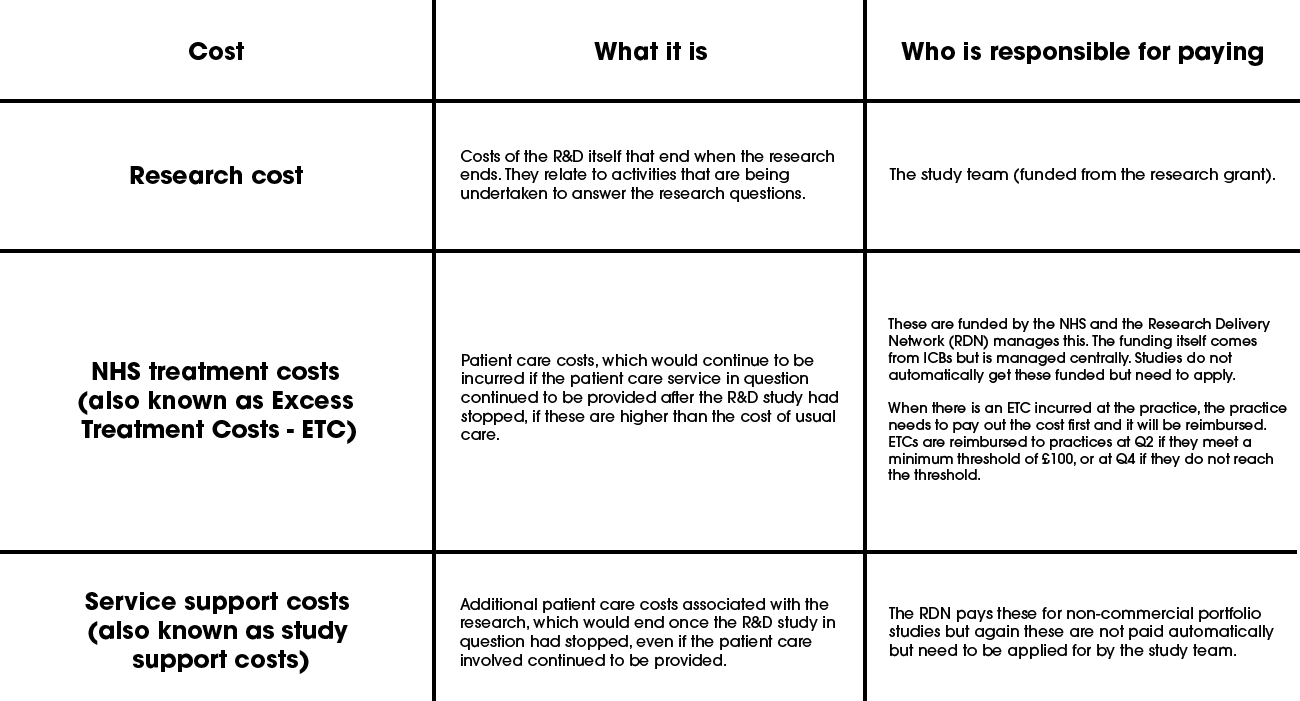
Practices should:
- obtain assurance that any costs incurred by the practice will be covered. Receiving an advisory email from the South West London R&D team will include assurance of this.
- Ensure that the necessary contracts are in place to enable practices to get paid.
If you want to read more about this, please see: Attributing the costs of health and social care research & development (publishing.service.gov.uk).
Obtaining the finance
The study team and RDN should provide information about how finance can be accessed relating to a study.
To obtain RDN funding, a practice needs a contract to be set up with the RDN. Contact [email protected] if you have any questions about contracts. Service support costs are paid quarterly in arrears, so it may be some time after the activity when the practice actually gets paid.
To obtain research funding, the practice may need to invoice the study team. The study team should provide the detail of how this is done. The contract/PIC agreement/Organisation Information Document (appendix 2) should detail what will be paid and how. The practice may need to add its bank details to one of the appendices in the contract.
For PIC activity, the funding may be paid by the research site as this is the organisation who has the contract with the practices.
Other finances
The RDN may occasionally offer additional funding to support practices with their research. There are regular funding opportunities and this will be updated when funding becomes available.
The RDN Agile Research Delivery Team also provides the opportunity for practices to request support to deliver specific studies – this can be applied for via https://sites.google.com/nihr.ac.uk/art/submit-a-request
Glossary of Acronyms and Terms
-
- CRN – Clinical Research Network
- GCP – Good Clinical Practice training
- OID – Organisation Information Document
- RDN – Research Delivery Network
- ICB – Integrated Care Board
- ETC – Excess Treatment Costs
- PIC – Participant Identification Centre
- OID – Organisation Information Document
Who pays
Research guidance is clear on which organisation is responsible for paying for certain things.
You will be given information on these, but the below is provided for information to aid understanding about who is responsible for what.

Practices should:
- obtain assurance that any costs incurred by the practice will be covered. Receiving an advisory email from the South West London R&D team will include assurance of this.
- Ensure that the necessary contracts are in place to enable practices to get paid.
If you want to read more about this, please see: Attributing the costs of health and social care research & development (publishing.service.gov.uk).
Research guidance is clear on which organisation is responsible for paying for certain things.
You will be given information on these, but the below is provided for information to aid understanding about who is responsible for what.

Practices should:
- obtain assurance that any costs incurred by the practice will be covered. Receiving an advisory email from the South West London R&D team will include assurance of this.
- Ensure that the necessary contracts are in place to enable practices to get paid.
If you want to read more about this, please see: Attributing the costs of health and social care research & development (publishing.service.gov.uk).
Obtaining the finance
The study team and RDN should provide information about how finance can be accessed relating to a study.
To obtain RDN funding, a practice needs a contract to be set up with the RDN. Contact [email protected] if you have any questions about contracts. Service support costs are paid quarterly in arrears, so it may be some time after the activity when the practice actually gets paid.
To obtain research funding, the practice may need to invoice the study team. The study team should provide the detail of how this is done. The contract/PIC agreement/Organisation Information Document (appendix 2) should detail what will be paid and how. The practice may need to add its bank details to one of the appendices in the contract.
For PIC activity, the funding may be paid by the research site as this is the organisation who has the contract with the practices.
The study team and RDN should provide information about how finance can be accessed relating to a study.
To obtain RDN funding, a practice needs a contract to be set up with the RDN. Contact [email protected] if you have any questions about contracts. Service support costs are paid quarterly in arrears, so it may be some time after the activity when the practice actually gets paid.
To obtain research funding, the practice may need to invoice the study team. The study team should provide the detail of how this is done. The contract/PIC agreement/Organisation Information Document (appendix 2) should detail what will be paid and how. The practice may need to add its bank details to one of the appendices in the contract.
For PIC activity, the funding may be paid by the research site as this is the organisation who has the contract with the practices.
Other finances
The RDN may occasionally offer additional funding to support practices with their research. There are regular funding opportunities and this will be updated when funding becomes available.
The RDN Agile Research Delivery Team also provides the opportunity for practices to request support to deliver specific studies – this can be applied for via https://sites.google.com/nihr.ac.uk/art/submit-a-request
Glossary of Acronyms and Terms
-
- CRN – Clinical Research Network
- GCP – Good Clinical Practice training
- OID – Organisation Information Document
- RDN – Research Delivery Network
- ICB – Integrated Care Board
- ETC – Excess Treatment Costs
- PIC – Participant Identification Centre
- OID – Organisation Information Document